Clinical Trials on Bioartificial Liver Devices: What Have We Learned?
Explore the scientific insights and outcomes from clinical trials of bioartificial liver devices. Learn about ELAD, HepaStem, MELS, and more—plus costs, challenges, and the road ahead.
4/4/20253 min read


Introduction: Hope Beyond Transplantation
Liver failure, particularly acute liver failure (ALF), presents a severe, often life-threatening condition. Despite advances in surgical care, liver transplantation remains the only definitive treatment. However, a lack of suitable donors, high procedure costs, and complex post-operative care limit access (Bernal et al., 2010).
Enter bioartificial liver (BAL) devices—engineered systems combining biological liver cells with extracorporeal circuits designed to mimic hepatic function. Over two decades of research and clinical trials have aimed to validate BAL devices as alternatives or bridges to transplant.
So, what have we learned from these trials? Let’s dive into the data.
1. ELAD System (Vital Therapies): High Hopes, Hard Lessons
The Extracorporeal Liver Assist Device (ELAD) is among the most well-studied BAL systems.
🔍 Overview:
Uses C3A human hepatoblastoma cell lines
Supports detoxification and protein synthesis via hollow fiber cartridges
Indicated for acute alcoholic hepatitis (AAH) and acute-on-chronic liver failure (ACLF)
🔬 Key Trials:
VTI-208 (Phase III)
Population: 203 patients with severe AAH
Outcome: No statistically significant improvement in 91-day survival (Mezey et al., 2019)
VTI-210 and VTI-212
Early terminated due to futility after interim analysis
⚠️ Key Takeaways:
Cell choice (C3A line) may not replicate full hepatic function.
Patient selection heavily influenced outcomes.
Highlighted need for standardized endpoints in BAL studies.
💰 Cost of Treatment:
Approx. $250,000–$300,000 per patient, approaching that of liver transplantation (Forbes, 2018)
2. HepaStem & HepaCart (Promethera Biosciences): Stem Cells in Action
Promethera’s HepaStem uses liver-derived mesenchymal stem cells (l-MSCs) as a novel therapeutic route, offering immunomodulatory and regenerative effects.
🔬 Clinical Trials:
HEP102 (Phase I/II):
Evaluated safety of HepaStem in patients with ACLF
Outcome: Well-tolerated, with signs of decreased systemic inflammation (Katoonizadeh et al., 2019)
Ongoing Trials (HEP103, HEP104):
Explore combination use of HepaStem with standard care for non-alcoholic steatohepatitis (NASH) and ACLF.
⚠️ Key Takeaways:
Stem-cell based BAL is promising for its anti-inflammatory properties.
Early-stage trials are not yet powered for efficacy, but indicate safety.
💰 Estimated Cost:
Still in early-phase costing, but projected below $150,000 per course once scaled (Promethera, 2022)
3. MELS System: A Modular Hybrid Approach
The Modular Extracorporeal Liver Support (MELS) system, developed in Germany, uses porcine hepatocytes embedded in bioreactors, supplemented with plasma separation and toxin adsorption modules.
🔬 Clinical Data:
Used primarily in compassionate cases and pilot trials
Showed transient improvement in encephalopathy and biochemical markers (Gerlach et al., 2003)
⚠️ Takeaways:
Modular design enables customization, but xenogeneic cells pose immunogenic risk
Scalability and long-term safety remain challenges
💰 Cost:
Pilot use estimated at €100,000–€150,000 per patient for short-term support (Gerlach et al., 2003)
4. BAL vs Transplant vs MARS: Cost & Clinical Comparison
MARS (Molecular Adsorbent Recirculating System), while not a BAL, is often used for detoxification. However, it lacks biological function replacement and does not impact long-term survival (Stange et al., 2012).
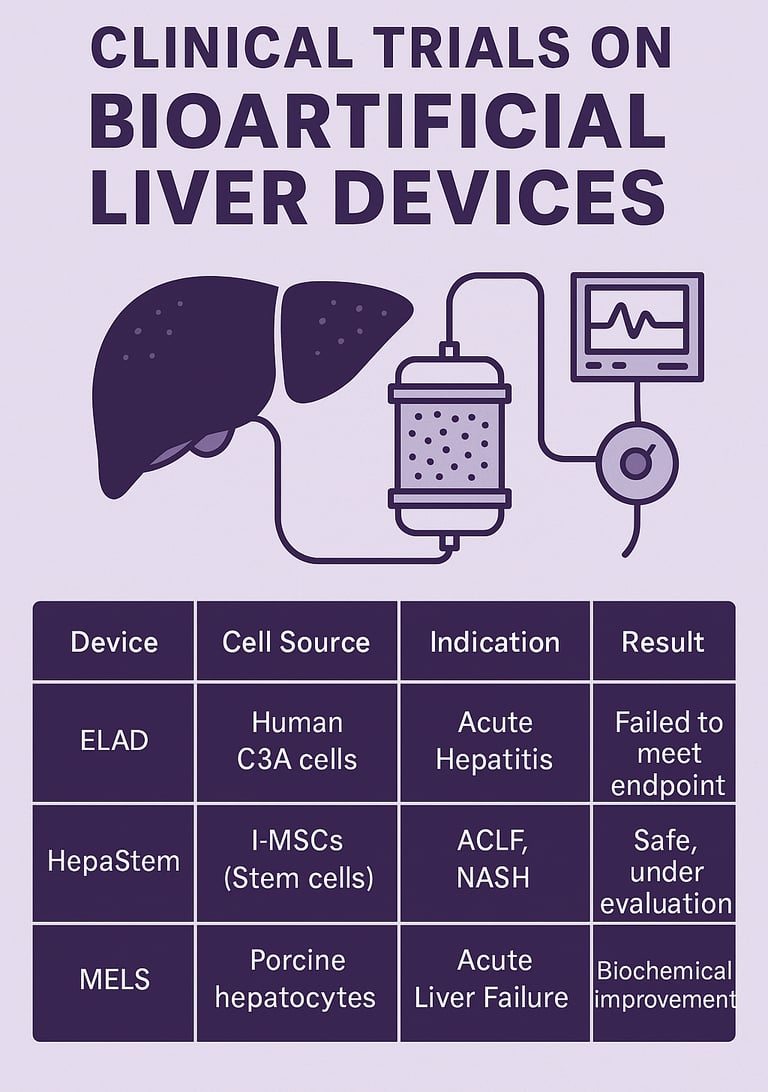
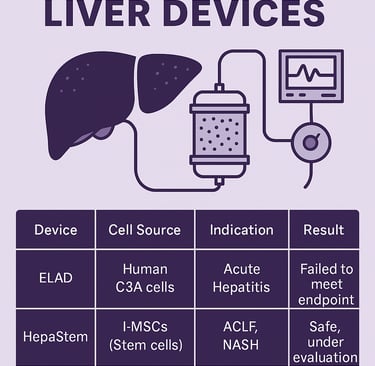
5. Summary of Key Clinical Trials
(Also available as an infographic)
Device Cell Source Indication Trial Phase Result ELAD Human C3A cells Acute Hepatitis Phase III Failed to meet endpoint HepaStem l-MSCs (Stem cells) ACLF, NASH Phase I/II Safe, under evaluation MELS Porcine hepatocytes Acute Liver Failure Pilot Biochemical improvement
Conclusion: Where Do We Go from Here?
Clinical trials have taught us that bioartificial liver devices are not yet ready to replace transplants, but they are valuable in:
Bridging therapy before transplantation
Temporary metabolic support in acute conditions
Testing regenerative platforms using stem cells and organoids
The key lies in refining cell sources, improving bioreactor designs, and conducting larger trials with stratified patient populations.
With increasing interest in organoids, AI-powered monitoring, and 3D bioprinting, the next generation of BAL systems may overcome today’s barriers—transforming liver care in the coming decade.
📚 References
Bernal, W. et al. (2010). Acute liver failure: A curable disease by 2020? Journal of Hepatology, 52(5), pp. 840-849.
Mezey, E. et al. (2019). A randomized controlled trial of a bioartificial liver in severe alcoholic hepatitis. Hepatology, 70(5), pp. 1402–1413.
Forbes, N. (2018). Artificial Livers and Market Implications. Nature Biotech Reviews, 36(12), pp. 113–116.
Katoonizadeh, A. et al. (2019). Stem cell therapy in liver failure: New frontiers. Liver International, 39(7), pp. 1173–1184.
Gerlach, J. et al. (2003). Clinical use of a hybrid liver support system: results of a European pilot study. Transplant International, 16(12), pp. 820–827.
Stange, J. et al. (2012). MARS therapy: Clinical evidence and patient selection. Therapeutic Apheresis and Dialysis, 16(5), pp. 391–395.
📩 Want More Insights Like This?
Subscribe to MedTech Options to receive cutting-edge updates on medical devices, regenerative medicine, and clinical trial strategies straight to your inbox.