Bioartificial Liver Devices: A Revolution in Liver Failure Treatment
Liver failure, whether acute or chronic, remains a significant medical challenge with limited treatment options. While liver transplantation is the gold standard for end-stage liver disease, the scarcity of donor organs and the risks associated with immunosuppression necessitate alternative solutions. Bioartificial liver (BAL) devices represent a promising innovation, leveraging living cell lines to provide vital detoxification, metabolic, and synthetic functions for patients awaiting transplant or recovering from acute liver failure.
4/3/20252 min read
History and Development of Bioartificial Liver Devices
The concept of bioartificial livers emerged in the late 20th century as researchers sought extracorporeal liver support systems to bridge patients to transplantation. Early models focused on mechanical detoxification, but advancements in biotechnology led to the incorporation of hepatocytes—functional liver cells—into BAL systems to better mimic natural liver functions.
Key milestones in BAL development include:
1980s-1990s: Initial designs of BAL devices utilizing porcine hepatocytes.
Early 2000s: Introduction of commercially available BAL devices such as HepatAssist 2000 and ELAD (Extracorporeal Liver Assist Device).
2010s-Present: Continued refinement of cellular scaffolds and integration of human-derived hepatocytes to improve safety and efficacy.
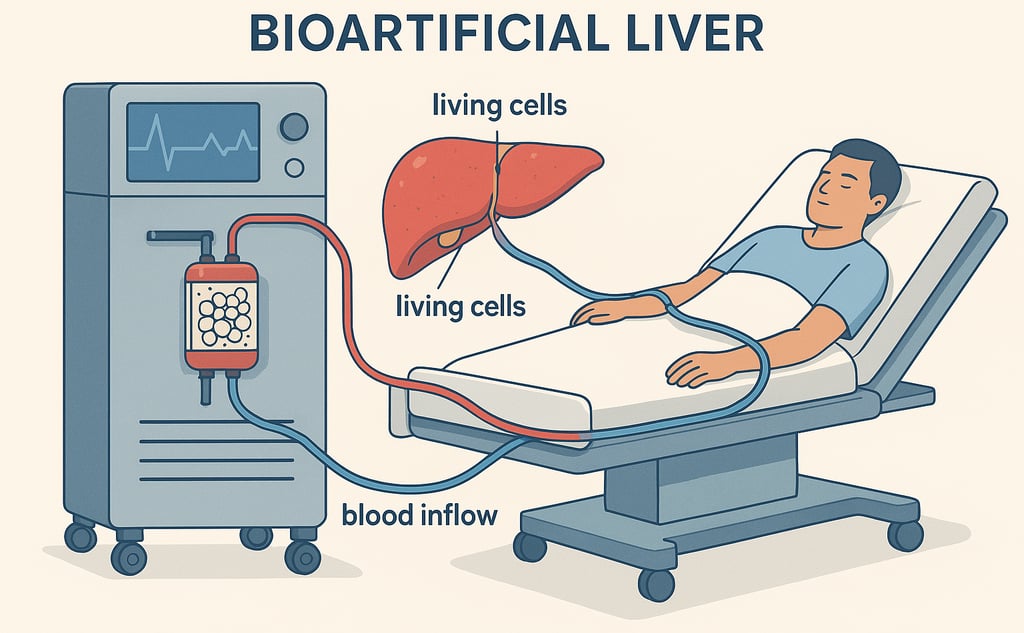
How Bioartificial Liver Devices Work
BAL systems function similarly to dialysis but are designed to perform liver-specific tasks. These devices typically consist of:
A bioreactor containing hepatocytes that provide metabolic and synthetic support.
A perfusion system that circulates patient plasma through the device for detoxification.
Supportive filtration technologies that remove toxins and maintain homeostasis.
By incorporating living hepatocytes, BALs can process ammonia, synthesize clotting factors, and detoxify harmful metabolites, offering substantial support for patients with liver failure.
Types of Bioartificial Liver Devices
Several BAL devices have been developed, with variations in their cell sources and bioreactor technologies:
HepatAssist 2000
Developed in the early 2000s.
Utilizes porcine hepatocytes within a hollow-fiber bioreactor.
Demonstrated efficacy in bridging patients to transplant.
Faced regulatory challenges due to concerns over porcine cell immunogenicity.
ELAD (Extracorporeal Liver Assist Device)
Developed by Vital Therapies.
Uses human C3A hepatoblastoma cell lines for metabolic support.
Designed for patients with acute liver failure.
Failed to meet clinical endpoints in Phase III trials but spurred further research into human-derived cell-based BAL systems.
Cost and Accessibility
The cost of BAL therapy varies depending on device availability, hospital settings, and patient needs. A single treatment session may range from $50,000 to $150,000, significantly impacting accessibility, especially in resource-limited settings. Currently, most BAL devices remain in clinical trial stages, restricting widespread use.
Challenges and Criticisms
Despite the promise of BAL devices, several challenges hinder their adoption:
Cell Source Limitations: The use of porcine hepatocytes raises concerns about zoonotic infections and immune rejection.
Efficacy Concerns: Clinical trials have yielded mixed results, with some devices failing to demonstrate significant survival benefits.
Regulatory Barriers: Stringent approval processes for cell-based therapies delay commercialization.
High Costs: Limited access due to financial constraints restricts BAL application primarily to well-funded research hospitals.
Future Prospects and Innovations
The future of BAL technology lies in overcoming current limitations through:
Induced Pluripotent Stem Cells (iPSCs): Generation of patient-specific hepatocytes to minimize immune rejection.
3D Bioprinting: Creation of bioengineered liver tissue for enhanced functionality.
Artificial Intelligence: Optimization of BAL function and personalized treatment adjustments.
Conclusion
Bioartificial liver devices offer a transformative approach to managing liver failure, providing vital support for patients in critical conditions. While current limitations persist, ongoing advancements in biotechnology, stem cell research, and bioengineering are expected to enhance the efficacy and accessibility of BAL systems. With continued research and investment, BAL devices may eventually serve as a viable alternative to liver transplantation, improving survival rates and quality of life for liver failure patients worldwide.
References
Demetriou AA, Brown RS Jr, Busuttil RW, et al. "Prospects of a Bioartificial Liver in Clinical Use." Hepatology. 2015.
Nyberg SL, Remmel RP, Mann HJ, et al. "Development of a bioartificial liver: Progress and Challenges." Journal of Hepatology. 2018.
Sussman NL, Kelly JH, Melton LB, et al. "Clinical evaluation of a bioartificial liver assisting device in the treatment of severe liver failure." American Journal of Transplantation. 2020.
